The Chemistry That Makes Cooked Food Tasty

I don't believe I'm alone in saying that crispy grilled sweet potato fries are delicious. And, though they might pale in comparison (I stand firm on this), normal fries can be pretty good, too - right alongside charred corn cobs, browned rice and oven-baked vegetables.
Now, I mention these foods because they are of particular importance to me at the moment, as I have been teaching myself recipes for them in an attempt to impress some university friends when I invite them over. (FYI - the results are promising.) I'm a bit of a scientist, however, so cooking vegetables quickly roused my curiosity. "Why do I have to dry the veggies in the fridge before I put them in the oven? Why do some online recipes recommend using baking soda on my french fries? Why is this so damn good?" I had to know.
There is truth in the saying that cooking is chemistry. Frying, baking, charring, caramelising - all of them are just sets of well-timed chemical reactions. But I have been focusing primarily on how bread becomes toast and such, and so I bring us to the lucrative concept of the Maillard reaction.
The Maillard Reaction
When food browns, it is almost always due to a chain of reactions occuring from a principal event. When two reactants join, they might produce an unstable compound that splits in a unique way, or one that accepts a couple extra hydrogen atoms in its molecular structure. This could then lead to multiple other molecules reacting in certain manners that bring about an even larger set of molecules - until the chain becomes exponentially more massive and messier over time. The Maillard reaction is like this, with its incindiary event being the joining of a peptide and a saccharide.
First of all, we must understand what peptides and saccharides really are, so here's some basic biochemistry for you. The former is another name for amino acids, which are monomers - building blocks that your body regularly uses to build proteins/polypeptides, which can simply be defined as sequences of amino acids. In total, there are 20 unique amino acids that naturally occur in the wild, but all of them have the same underlying core - a carbon atom (C) bonded to a carboxyl group (-COOH), an amine group (-NH2), a hydrogen atom (H) and what is known as an R-group (which is the part that differs between different amino acids).
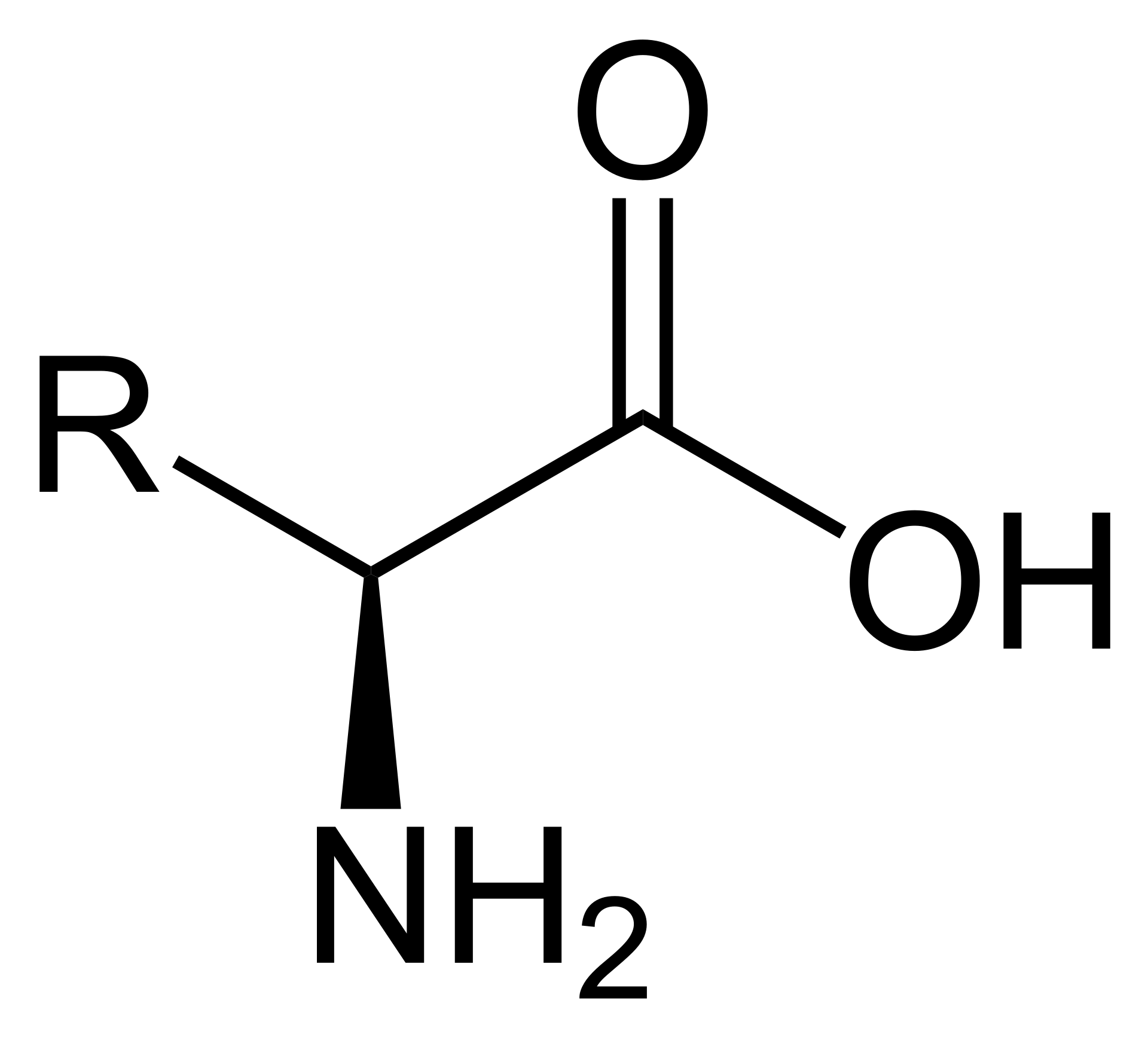
In comparison, saccharides - which are simply units or polymer sequences of monosaccharides like glucose, fructose or galactose bonded together - have no amine groups whatsoever. They do contain carbonyl groups, though - and here is where we observe most of their chemical reactions taking place.
To start the Maillard reaction, a saccharide's carbonyl group must react with the amine group of a peptide in a condensation reaction (which produces water as a waste product). This can be encouraged by making conditions alkaline by adding lye or baking soda to the concoction, as this prevents the amine group from reacting with other substances around it (like water). Then, when the molecules bond, the reaction produces what is referred to as an N-substituted glycosylamine. (The 'N-substituted' part only tells you that a nitrogen atom is holding the molecule together, with 'glycos' referring to the sugar and 'amine' speaking of, well, the amino acid!) This step is crucial, as every other reaction that follows is derived from the glycosalamine changing shape (i.e. isomerising) to create ketosamine molecules.
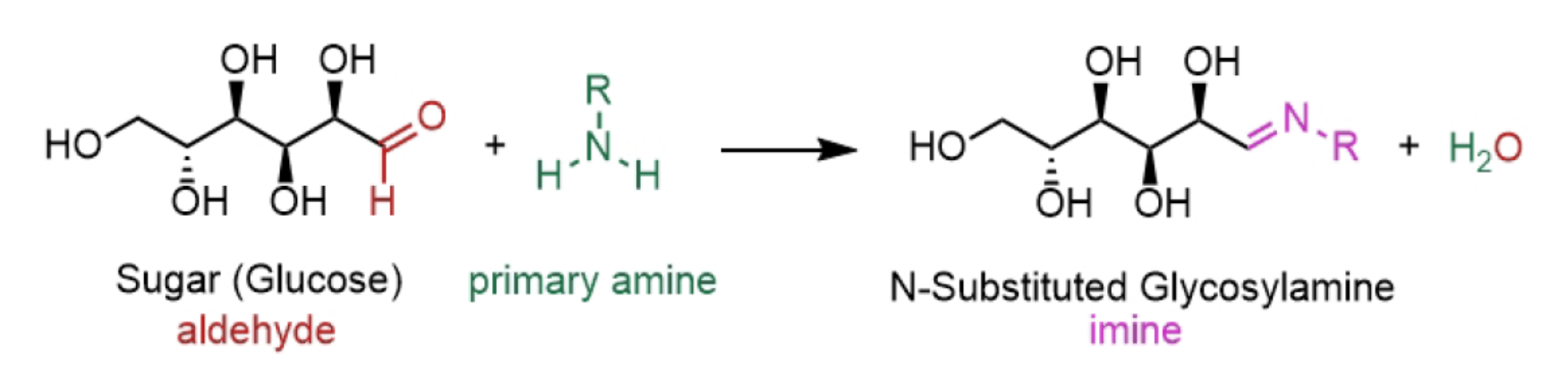
So, now that we're comfortably aware of the proceedings, how does this all link back to cooking?
Hot, Hotter And Hottest
Let's imagine that we are grilling a fish - a grouper, perhaps, because I rather like those. We set the grill to a temperature of approximately 150ºC, add some salt and lemon to the grouper on both sides and then place it on the grill for enough time to turn one side crispy brown. We flip the fish over, and continue until it is cooked satisfactorily well throughout and the tantalising smell is intoxicating me. Lovely. Notice, the temperature we used is several times higher than the grouper's normal body temperature would have been. This is done on purpose, of course, as we want to brown the fish - because the Maillard reaction can take place at 150ºC in dry conditions - and kill any inhabiting bacteria on it.

This also tells us that the Maillard reaction is non-enzymatic, meaning that the chemical reactions involved in it are not catalyzed by enzymes. This makes sense, as enzymes can only work in optimum temperature conditions (the grouper's body temperature, in this case), breaking down when it gets too hot. As such, we conclude that all the reactions in the process must transpire due to the heat energy alone - which is exactly right. Even the first reaction - when the peptide and saccharide bond together - is a result of the heat energising the reactant molecules so that they can move faster and collide with sufficient kinetic energy to merge.
Later products in the chain have similar origins. The aforementioned ketosamines could break into smaller pieces, for instance, due to the heat breaking some bonds inside of it (a process called pyrolysis). In fact, whether they break down or join together or change shape, all the molecules in the Maillard reaction can only continue reacting in the presence of a suitable amount of heat. Depending on the temperature you use when cooking (in the range of 60-180ºC), you could even influence the resulting products that end up in your food - though it admittedly depends even more on the sugars and proteins that the ingredients contained before being cooked (so you can't get the same flavour from grilled corn, for example, as you would from grilled pepper, even when cooked at the same temperature).

Interestingly, there have been studies in the past that attempted to find more accurate and measurable ways to control the rate and products of Maillard reactions. One of these, made by a group of scientists in the American Chemical Society, even proposed using pulsed electric fields and high pressure conditions to avoid forming some high-heat products. Not very feasible as a cooking strategy, I think, but interesting nonetheless.
Do note that the Maillard is different from caramelisation, as the latter has to do with sugars being oxidised to form other sugars, with no amino acids appearing in the process. The same goes for burning food - at high enough temperatures, Maillard reactants will stop reacting together and will begin combusting spontaneously with the oxygen in the air, thereby turning the food from brown to black (I swear this has never happened to me). Confusingly, these three phenomena can also happen simultaneously, leaving you with a strange spectacle of burning, caramelising and Maillard reaction-ing. Either way, to stop food from burning, always make sure to be responsibly focused and to cook at appropriate temperatures!
Cooking Adaptations
Why does cooked food taste so good? Why does the average person shirk at the thought of slurping raw egg whites, but brightens up at the mention of fried eggs? Apparently, the answer lies in our gut.
Though we are capable of digesting and absorbing most animal-based proteins, a lot of the time, it is an honest struggle for our bodies to do so. An egg's proteins, for instance, might be too soluble and too slippery for our protein-cutting enzymes (peptidases) to easily function on. In this case, cooking the egg outside of the body proves to be surprisingly helpful. The heat breaks down intermolecular bonds within the proteins and essentially makes them unfold, losing their solubility and solidifying the egg into a form that is more easily digestible for when we consume it. This allows the body to conserve energy from digestion, and makes the experience generally easier for us.

As it turns out, the body's quite fond of easy things in life, and so it rewards you for experiencing them; millions of years of evolution have only made this all the more prominent. In terms of food, we are rewarded by the tastes and smells that cooking food brings us. Maillard reaction products like pyrazines, pyrroles, furans and oxazoles are all organoleptic (stimulating sensory organs) and contribute to the flavour and colouring of food, and they come right alongside the easily digestible nutrients that I talked about. It all comes from cooking.
Furthermore, studies have shown that leaving some foods at the right temperatures for some time can actually raise nutritional values, like in their antioxidant levels. Awkwardly, I am not completely sure about how this can come about, but it still serves to explain why humans can be so attracted to things like black garlic (instead of regular garlic).
I won't lie, I do prefer eating cooked food over regular food, but I think my university friends will appreciate that about me. It's nice to see the chemistry behind it, though - particularly as you can imagine what's happening at the molecular level when making french fries!
References
- Compound Interest (2015). Food Chemistry - The Maillard Reaction. Compound Chemistry. Retrieved from https://www.compoundchem.com/2015/01/27/maillardreaction/
- Lund, M. N. & Ray, C. A. (2017). Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. Journal of Agricultural and Food Chemistry. Retrieved from https://pubs.acs.org/doi/10.1021/acs.jafc.7b00882
- Choi, I. S. et al (2014). Physicochemical and antioxidant properties of black garlic. Molecules. Retrieved from https://pubmed.ncbi.nlm.nih.gov/25335109/
